| General Information | |
|---|---|
| ZINC ID/ Molecule Name | ZINC000013683962 |
| Molecular Weight (Da) | 424 |
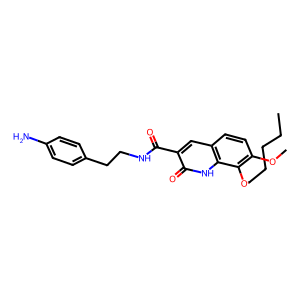
| SMILES | CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(N)cc3)c(=O)[nH]c12 |
| Molecular Formula | C24N3O4 |
| Action | Inverse Agonist |

| General Information | |
|---|---|
| ZINC ID/ Molecule Name | ZINC000013683962 |
| Molecular Weight (Da) | 424 |
| SMILES | CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(N)cc3)c(=O)[nH]c12 |
| Molecular Formula | C24N3O4 |
| Action | Inverse Agonist |
| Physicochemical Details | |
|---|---|
| ZINC ID/ Molecule Name | ZINC000013683962 |
| Molar Refractivity | 122.322 |
| HBA | 4 |
| HBD | 3 |
| Rotatable Bonds | 10 |
| Heavy Atoms | 31 |
| LogP | 3.831 |
| Activity (Ki) in nM | 3.162 |
| Polar Surface Area (PSA) | 106.44 |
| Pharmacokinetic Properties | |
|---|---|
| ZINC ID/ Molecule Name | ZINC000013683962 |
| Human intestinal absorption | + |
| Caco-2 | - |
| Blood brain barrier | + |
| P-glycoprotein inhibitior | + |
| P-glycoprotein substrate | + |
| Cyp3a4 substrate | + |
| Cyp2c9 substrate | - |
| Cyp2d6 substrate | - |
| Cyp3a4 inhibition | + |
| Cyp2c9 inhibition | - |
| Cyp2c19 inhibition | - |
| Cyp2d6 inhibition | - |
| Cyp1a2 inhibition | - |
| Oatp2b1 inhibitor | - |
| Oatp1b1 inhibitor | + |
| Oatp1b3 inhibitor | + |
| Mate1 inhibitor | - |
| Oct2 inhibitor | - |
| Bsep inhibitor | + |
| Acute oral toxicity | - |
| Carcinogenicity (binary) | - |
| Ames mutagenesis | - |
| Human ether-a-go-go-related gene inhibition | + |
| Biodegradation | |
| Glucocorticoid receptor binding | + |
| Thyroid receptor binding | + |
| Androgen receptor binding | + |
| Plasma protein binding | 0.83122319 |
| Pharmacokinetic Properties | |
|---|---|
| Number of aromatic heavy atoms | 16 |
| Fraction csp3 | 0.33 |
| Ilogp | 3.95 |
| Xlogp3 | 4.38 |
| Wlogp | 3.67 |
| Mlogp | 2.38 |
| Silicos-it log p | 4.98 |
| Consensus log p | 3.87 |
| Esol log s | -4.88 |
| Esol solubility (mg/ml) | 5.57E-03 |
| Esol solubility (mol/l) | 1.32E-05 |
| Esol class | Moderately |
| Ali log s | -6.33 |
| Ali solubility (mg/ml) | 1.97E-04 |
| Ali solubility (mol/l) | 4.66E-07 |
| Ali class | Poorly sol |
| Silicos-it logsw | -8.35 |
| Silicos-it solubility (mg/ml) | 1.89E-06 |
| Silicos-it solubility (mol/l) | 4.46E-09 |
| Silicos-it class | Poorly soluble |
| Pgp substrate | |
| Log kp (cm/s) | -5.77 |
| Lipinski number of violations | 0 |
| Ghose number of violations | 0 |
| Veber number of violations | 1 |
| Egan number of violations | 0 |
| Muegge number of violations | 0 |
| Bioavailability score | 0.55 |
| Pains number of alerts | 1 |
| Brenk number of alerts | 1 |
| Leadlikeness number of violations | 3 |
| Synthetic accessibility | 3.24 |
| Pharmacokinetic Properties | |
|---|---|
| Logs | -4.547 |
| Logd | 3.268 |
| Logp | 3.627 |
| F (20%) | 0.005 |
| F (30%) | 0.026 |
| Mdck | 1.73E-05 |
| Ppb | 0.9718 |
| Vdss | 0.883 |
| Fu | 0.0169 |
| Cyp1a2-inh | 0.78 |
| Cyp1a2-sub | 0.601 |
| Cyp2c19-inh | 0.904 |
| Cyp2c19-sub | 0.102 |
| Cl | 5.23 |
| T12 | 0.227 |
| H-ht | 0.638 |
| Dili | 0.831 |
| Roa | 0.195 |
| Fdamdd | 0.736 |
| Skinsen | 0.253 |
| Ec | 0.003 |
| Ei | 0.012 |
| Respiratory | 0.35 |
| Bcf | 0.757 |
| Igc50 | 4.005 |
| Lc50 | 5.056 |
| Lc50dm | 6.181 |
| Nr-ar | 0.027 |
| Nr-ar-lbd | 0.004 |
| Nr-ahr | 0.901 |
| Nr-aromatase | 0.907 |
| Nr-er | 0.336 |
| Nr-er-lbd | 0.009 |
| Nr-ppar-gamma | 0.134 |
| Sr-are | 0.735 |
| Sr-atad5 | 0.85 |
| Sr-hse | 0.413 |
| Sr-mmp | 0.703 |
| Sr-p53 | 0.829 |
| Vol | 442.414 |
| Dense | 0.957 |
| Flex | 19 |
| Nstereo | 0.579 |
| Nongenotoxic carcinogenicity | 0 |
| Ld50 oral | 0 |
| Genotoxic carcinogenicity mutagenicity | 0 |
| Surechembl | 5 |
| Nonbiodegradable | 0 |
| Skin sensitization | 1 |
| Acute aquatic toxicity | 3 |
| Toxicophores | 0 |
| Qed | 2 |
| Synth | 0.341 |
| Fsp3 | 2.314 |
| Mce-18 | 0.333 |
| Natural product-likeness | 18 |
| Alarm nmr | -0.552 |
| Bms | 3 |
| Chelating | 0 |
| Pfizer | 3 |
| Gsk | Accepted |
| Goldentriangle | Rejected |